ISO 10993-7 2008 Amd 1 2019 医疗器械生物学评价--第7部分环氧乙烷灭菌残留 修正案1适用性新生儿 和 婴儿 的 允许 限度(中英文)
INTERNATIONAL
STANDARD
ISO
10993-7
Second edition
2008-10-15
AMENDMENT 1
2019-12
Biological evaluation of medical
devices —
Part 7:
Ethylene oxide sterilization residuals
AMENDMENT 1:Applicability of
allowable limits for neonates and infants
Reference number
ISO 10993-7:2008/Amd.1:2019(E)
C ISO 2019
国际标准 ISO
10993-7
第 二 版
2008-10-15
修正案1
2019-12
医疗器械生物学评价——
第7部分:
环氧乙烷灭菌残留
修正案1:适用性
新生儿 和 婴儿 的 允许 限度
参考编号
ISO 10993-7:2008/Amd. 1:2019(E)
C ISO 2019
ISO 10993-7:2008/Amd.1:2019(E)
COPYRIGHT PROTECTED DOCUMENT
C ISO 2019
All rights reserved.Unless otherwise specified,or required in the context of its implementation,no part of this publication may
be reproduced or utilized otherwise in any form or by any means,electronic or mechanical,including photocopying,or posting
on the internet or an intranet,without prior written permission.Permission can be requested from either ISO at the address
below or ISO's member body in the country of the requester.
ISO copyright office
CP 401·Ch.de Blandonnet 8
CH-1214 Vernier,Geneva
Phone:+41227490111
Fax:+41227490947
Email:copyright@iso.org
Website:www.iso.org
Published in Switzerland
ii CISO 2019-All rights reserved
摘要:
展开>>
收起<<
INTERNATIONALSTANDARDISO10993-7Secondedition2008-10-15AMENDMENT12019-12Biologicalevaluationofmedicaldevices—Part7:EthyleneoxidesterilizationresidualsAMENDMENT1:ApplicabilityofallowablelimitsforneonatesandinfantsReferencenumberISO10993-7:2008/Amd.1:2019(E)CISO2019国际标准ISO10993-7第二版2008-10-15修正案12019-1...
声明:如果您的权利被侵害,请联系我们的进行举报。
相关推荐
-
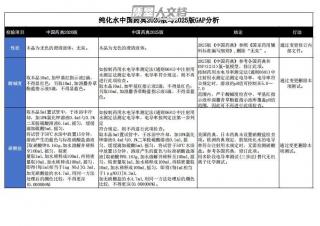
纯化水中国药典2020版与2025版GAP分析VIP免费
 2025-04-18 308
2025-04-18 308 -
无菌检查法对比表(2025版药典 VS 2020版药典)

 2025-09-27 375
2025-09-27 375 -
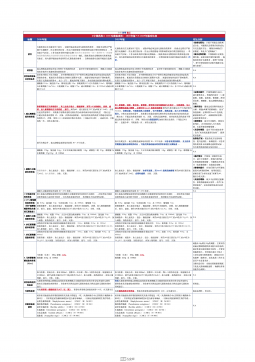
1101无菌检查法对比表(2025版药典 VS 2020版药典)
 2025-09-29 278
2025-09-29 278 -
1143 细菌内毒素检查法对比表(2025版药典 VS 2020版药典)

 2025-09-29 614
2025-09-29 614 -
ECA-污染控制策略指南(中英文)-202202VIP免费

 2025-11-04 62
2025-11-04 62 -
TR-26-2025-Sterilizing-Filtration-of-Liquids液体的灭菌过滤(中英文对照版)VIP专享

 2025-11-06 393
2025-11-06 393 -
TR-26-2025-Sterilizing-Filtration-of-Liquids液体的灭菌过滤(英文版)VIP免费

 2025-11-06 123
2025-11-06 123 -
PDA-TR49-生物清洁验证-中英文翻译

 2025-11-19 79
2025-11-19 79 -
硫酸艾玛昔替尼片(CXHS2300097)说明书VIP免费

 2025-11-26 167
2025-11-26 167 -
达格列净片说明书VIP免费

 2026-01-13 23
2026-01-13 23
作者: 51zlzl
分类:法规规范
价格:300质量币
属性:18 页
大小:655.83KB
格式:PDF
时间:2025-11-21


