ISO TS 10993-20 2006 医疗器械生物学评价第20部分医疗器械免疫毒理学试验的原则与方法(中英文)
TECHNICAL
SPECIFICATION
ISO/TS
10993-20
First edition
2006-08-01
Biological evaluation of medical
devices—
Part 20:
Principles and methods for
immunotoxicology testing of medical
devices
Evaluation biologique des dispositifs médicaux—
Partie 20:Principes et méthodes relatifs aux essais
d'immunotoxicologie des dispositifs médicaux
Reference number
ISO/TS 10993-20:2006(E)
◎ISO 2006
技术规格 ISO/TS
10993-20
第 一 版
2006-08-01
医疗器械生物学评价
第20部分:
医疗器械免疫毒性试验的原则和方法
医疗器械生物学评价
第
20
部分:医疗器械免疫毒理学试验的原则与方法
参 考 编 号 ISO/TS
10993-20:2006(E)
◎ISO 2006
ISO/TS 10993-20:2006(E)
PDF disclaimer
This PDF file may contain embedded typefaces.In accordance with Adobe's licensing policy,this file may be printed or viewed but
shall not be edited unless the typefaces which are embedded are licensed to and installed on the computer performing the editing.In
downloading this file,parties accept therein the responsibility of not infringing Adobe's licensing policy.The ISO Central Secretariat
accepts no liability in this area.
Adobe is a trademark of Adobe Systems Incorporated.
Details of the software products used to create this PDF file can be found in the General Info relative to the file;the PDF-creation
parameters were optimized for printing.Every care has been taken to ensure that the file is suitable for use by ISO member bodies.In
the unlikely event that a problem relating to it is found,please inform the Central Secretariat at the address given below.
◎ISO 2006
AIl ights reserved.Unless otherwise specified,no part of this publication may be reproduced or utilized in any form or by any means,
electronic or mechanical,including photocopying and microfilm,without permission in writing from either ISO at the address below or
ISO's member body in the country of the requester
ISO copyright office
Case postale 56·CH-1211 Geneva 20
Tel.+41227490111
Fax +41227490947
E-mail copyright@iso.org
Web www.iso.org
Published in Switzerland
ii ◎ISO 2006-All rights reserved
摘要:
展开>>
收起<<
TECHNICALSPECIFICATIONISO/TS10993-20Firstedition2006-08-01Biologicalevaluationofmedicaldevices—Part20:PrinciplesandmethodsforimmunotoxicologytestingofmedicaldevicesEvaluationbiologiquedesdispositifsmédicaux—Partie20:Principesetméthodesrelatifsauxessaisd'immunotoxicologiedesdispositifsmédicauxReferen...
声明:如果您的权利被侵害,请联系我们的进行举报。
相关推荐
-
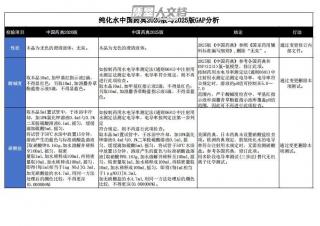
纯化水中国药典2020版与2025版GAP分析VIP免费
 2025-04-18 308
2025-04-18 308 -
无菌检查法对比表(2025版药典 VS 2020版药典)

 2025-09-27 375
2025-09-27 375 -
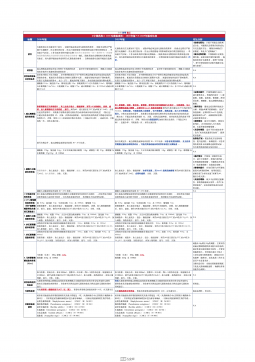
1101无菌检查法对比表(2025版药典 VS 2020版药典)
 2025-09-29 278
2025-09-29 278 -
1143 细菌内毒素检查法对比表(2025版药典 VS 2020版药典)

 2025-09-29 614
2025-09-29 614 -
ECA-污染控制策略指南(中英文)-202202VIP免费

 2025-11-04 62
2025-11-04 62 -
TR-26-2025-Sterilizing-Filtration-of-Liquids液体的灭菌过滤(中英文对照版)VIP专享

 2025-11-06 393
2025-11-06 393 -
TR-26-2025-Sterilizing-Filtration-of-Liquids液体的灭菌过滤(英文版)VIP免费

 2025-11-06 123
2025-11-06 123 -
PDA-TR49-生物清洁验证-中英文翻译

 2025-11-19 79
2025-11-19 79 -
硫酸艾玛昔替尼片(CXHS2300097)说明书VIP免费

 2025-11-26 167
2025-11-26 167 -
达格列净片说明书VIP免费

 2026-01-13 23
2026-01-13 23
作者: 51zlzl
分类:法规规范
价格:300质量币
属性:48 页
大小:1.04MB
格式:PDF
时间:2025-11-21


