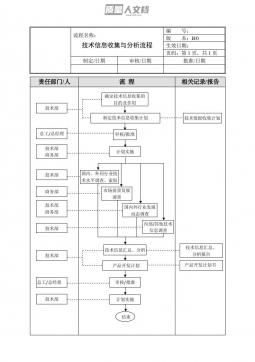
ISO 17665-2024 医疗器械湿热灭菌工艺的开发、 验证和常规控制要求 医疗保健产品灭菌 湿热灭菌要求
VIP免费
Reference number
Sterilization of health care
products—Moist heat—
Requirements for the development,
validation and routine control of
a sterilization process for medical
devices
Stérilisation des produits de santé—Chaleur humide—
Exigences pour le développement,la validation etle controle de
routine d'un procédé de stérilisation des dispositifs médicaux
ISO 17665:2024(en)
International
Standard
ISO 17665
First edition
2024-03
◎ISO 2024
ii
ISO 17665:2024(en)
COPYRIGHT PROTECTED DOCUMENT
◎ISO 2024
Allrights reserved.Unless otherwise specified,or required in the context of its implementation,no part of this publication may
be reproduced or utilized otherwise in any form or by any means,electronic or mechanical,including photocopying,or posting on
the internet or an intranet,without prior written permission.Permission can be requested from either ISO at the address below
or ISO's member body in the country ofthe requester:
ISO copyright office
CP 401·Ch.de Blandonnet 8
CH-1214 Vernier,Geneva
Phone:+41227490111
Email:copyright@iso.org
Website:www.iso.org
Published in Switzerland
◎ISO 2024-All rights reserved
iii
ISO 17665:2024(en)
Contents Page
Forewor ...................................................................................................................................................................................... V
Introduction ........................................................................................................................................................................... vi
1 Scope ...................................................................................................................................................................................
1
1.1 Inclusions ........................................................................................................................................................... 1
1.2 Exclusions ...........................................................................................................................................................
1
2 Normative references ............................................................................................................................................. 2
3 Terms and definitions ...............................................................................................................................................
2
4 Genera ....................................................................................................................................................................... 12
5 Sterilizing agent characterization ..................................................................................................................... 13
5.1 Sterilizing agen ............................................................................................................................................... 13
5.2 Microbicidal effectivenes .............................................................................................................................14
5.3 Effects on materials ...................................................................................................................................... 14
5.4 Environmental consideration ..................................................................................................................... 14
6 Process and equipment characterization ..................................................................................................... 14
6.1 Genera .............................................................................................................................................................14
6.2 Process characterization ..............................................................................................................................14
6.3 Saturated steam sterilization processes ................................................................................................. 15
6.4 Contained product sterilization processes ........................................................................................... 16
6.5 Equipmen ...........................................................................................................................................................17
7 Product definition ................................................................................................................................................... 18
8 Process definition ..................................................................................................................................................... 20
9 Validation .....................................................................................................................................................................22
9.1 Genera ........................................................................................................................................................... 22
9.2 Installation qualification (IQ) .................................................................................................................. 23
9.3 Operational qualification (0Q ................................................................................................................... 23
9.4 Performance qualification (PQ) .............................................................................................................. 24
9.5 Review and approval of validation ........................................................................................................... 26
10 Routine monitoring and contro .........................................................................................................................26
10.1 Routine monitoring ....................................................................................................................................... 26
10.2 Operational status ......................................................................................................................................... 26
10.3 Process verification .......................................................................................................................................27
10.4 Evaluation of additional data for saturated steam sterilization processes ...................................
27
10.5 Evaluation of additional data for contained product sterilization processes ..............................27
10.6 Record retention ........................................................................................................................................... 28
11 Product release from sterilization ................................................................................................................... 28
12 Maintaining process effectivenes ..................................................................................................................... 28
12.1 Purpose .......................................................................................................................................................... 28
12.2 Demonstration of continued effectivenes ............................................................................................... 28
12.3 Recalibration ................................................................................................................................................... 29
12.4 Equipment maintenance ........................................................................................................................... 29
12.5 Requalification ............................................................................................................................................. 29
12.6 Assessment of change ..................................................................................................................................30
Annex A (informative ) Guidance on the principles of moist heat sterilization and rationales for
requirements ........................................................................................................................................................... 31
Annex B (informative ) Establishment and evaluation of a sterilization process primarily based
on microbiological inactivation ........................................................................................................................... 59
Annex C( informative ) Establishment and evaluation of a sterilization process primarily based
on the measurement of physical parameters .............................................................................................. 73
◎ISO 2024-All rights reserved
摘要:
展开>>
收起<<
ReferencenumberSterilizationofhealthcareproducts—Moistheat—Requirementsforthedevelopment,validationandroutinecontrolofasterilizationprocessformedicaldevicesStérilisationdesproduitsdesanté—Chaleurhumide—Exigencespourledéveloppement,lavalidationetlecontrolederoutined'unprocédédestérilisationdesdisposi...
声明:如果您的权利被侵害,请联系我们的进行举报。
相关推荐
作者:薛定谔的龙猫
分类:法规规范
价格:60质量币
属性:164 页
大小:1.67MB
格式:PDF
时间:2025-11-13