ISO 10993-14:2001 医疗器械生物学评价 第14部分陶瓷降解产物的鉴定与定量(中英文)
INTERNATIONAL
STANDARD
ISO
10993-14
First edition
2001-11-15
Biological evaluation of medical devices—
Part 14:
Identification and quantification of
degradation products from ceramics
Évaluation biologique des dispositifs médicaux—
Partie 14:Identification et quantification des produits de dégradation des
céramiques
Reference number
ISO 10993-14:2001(E)
◎ISO 2001
Copynight Intemational Organization for Standardization
Provided by IHSunder license with iSO
No reproducfion or networking pernitted without license from IHS Not for Resale
国际标准 ISO
10993-14
第 一 版
2001-11-15
医疗器械生物学评价 第14部分:
陶瓷降解产物的鉴定与定量
医疗器械生物学评价
第
14
部分:陶瓷降解产物的鉴定与定量分析
参 考 编 号 ISO
10993-14:2001(E)
◎ISO 2001
国际标准化组织
由IHS根据iSO许可证提供
未经IHS授权,不得进行复制或网络共享 禁止转售
ISO 10993-14:2001(E)
PDF disclaimer
This PDF file may contain embedded typefaces.In accordance with Adobe's licensing policy,this file may be printed or viewed but shall not
be edited unless the typefaces which are embedded are licensed to and installed on the computer performing the editing.In downloading this
file,parties accept therein the responsibility of not infinging Adobe's licensing policy.The ISO Central Secretariat accepts no liability in this
area.
Adobe is a trademark of Adobe Systems Incorporated.
Details of the software products used to create this PDF file can be found in the General Info relative to the file;the PDF-creation parameters
were optimized for printing.Every care has been taken to ensure that the file is suitable for use by ISO member bodies.In the unlikely event
that a problem relating to it is found,please inform the Central Secretariat at the address given below.
◎ISO 2001
All rights reserved.Unless otherwise specified,no part of this publication may be reproduced or utilized in any form or by any means,electronic
or mechanical,including photocopying and microfilm,without permission in writing from either ISO at the address below or ISO's member body
in the country of the requester.
ISO copyright office
Case postale 56·CH-1211 Geneva 20
Tel.+41227490111
Fax +41227490947
Web www.iso.ch
Printed in Switzerland
Copyrant iemational Organizaton or Standardzation ◎ISO 2001-All rights reserved
Provided by IHSunder license with iSO
No reproducfion or networking pernitted without license from IHS Not for Resale
E-mail copyright@iso.ch
摘要:
展开>>
收起<<
INTERNATIONALSTANDARDISO10993-14Firstedition2001-11-15Biologicalevaluationofmedicaldevices—Part14:IdentificationandquantificationofdegradationproductsfromceramicsÉvaluationbiologiquedesdispositifsmédicaux—Partie14:IdentificationetquantificationdesproduitsdedégradationdescéramiquesReferencenumberISO1...
声明:如果您的权利被侵害,请联系我们的进行举报。
相关推荐
-
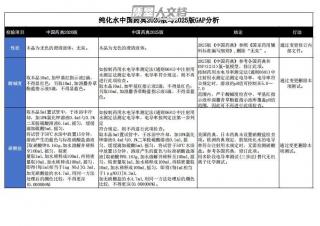
纯化水中国药典2020版与2025版GAP分析VIP免费
 2025-04-18 308
2025-04-18 308 -
无菌检查法对比表(2025版药典 VS 2020版药典)

 2025-09-27 375
2025-09-27 375 -
1101无菌检查法对比表(2025版药典 VS 2020版药典)
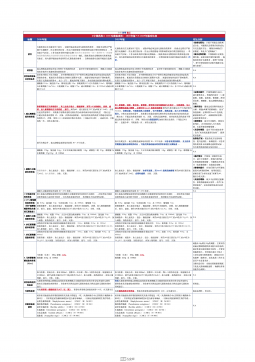
 2025-09-29 278
2025-09-29 278 -
1143 细菌内毒素检查法对比表(2025版药典 VS 2020版药典)

 2025-09-29 614
2025-09-29 614 -
ECA-污染控制策略指南(中英文)-202202VIP免费

 2025-11-04 62
2025-11-04 62 -
TR-26-2025-Sterilizing-Filtration-of-Liquids液体的灭菌过滤(中英文对照版)VIP专享

 2025-11-06 393
2025-11-06 393 -
TR-26-2025-Sterilizing-Filtration-of-Liquids液体的灭菌过滤(英文版)VIP免费

 2025-11-06 123
2025-11-06 123 -
PDA-TR49-生物清洁验证-中英文翻译

 2025-11-19 79
2025-11-19 79 -
硫酸艾玛昔替尼片(CXHS2300097)说明书VIP免费

 2025-11-26 167
2025-11-26 167 -
达格列净片说明书VIP免费

 2026-01-13 23
2026-01-13 23
作者: 51zlzl
分类:法规规范
价格:300质量币
属性:36 页
大小:957.2KB
格式:PDF
时间:2025-11-21