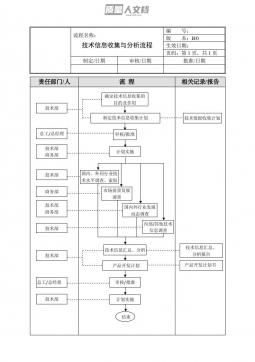
FDA证明生物类似药与参照药相似性的科学考量评估开展比较疗效研究必要性的最新建议
VIP免费
60 https://www.regulations.gov
HFA-305
5630 1061 20852
CDER
855 543-3784 301-796-2400CBER
800-835-4709 240-402-8010
CDER
CBER
2025 10
855-543-3784 301-796-3400
druginfo@fda.hhs.gov
https://www.fda.gov/drugs/guidance-compliance-regulatory-information/guidances-drugs
/
800-835-4709 240-402-8010
industry.biologics@fda.hhs.gov
https://www.fda.gov/vaccines-blood-biologics/guidance-compliance-regulatory-information-biologics/biologics-guidances
CDER
CBER
2025 10
DraftNot for Implementation
Contains Nonbinding Recommendations
. ........................................................................................................................................ 1
II. ....................................................................................................................................... 3
摘要:
展开>>
收起<<
furiOoNSqgovOvyfO0UkueHxzvvgeLNcSWIhHcSWgceNNOlBaNKu0QsNgIhHeNvaTW0TQlb0SwIhHcSWSOgNKew60YQcN0u5PacNhttpswwwregulationsgov0NfbaToTvwcthchHtRQlHFA305W0WlQpWQK95630S1061208520bgaWGlf0TQlb0SvSuwNbRvhchHS0YgIhHeNgNOUuTCDERoTOoRQlYu58555433784b3017962400bCBER0YTNSRQlu58008354709b24040280100VSkuNQlOgRToTv...
声明:如果您的权利被侵害,请联系我们的进行举报。
相关推荐
作者:薛定谔的龙猫
分类:专业资料
价格:60质量币
属性:7 页
大小:1.5MB
格式:PDF
时间:2025-11-11