fees-review-medical-device-licence-applications-2019-document-eng 医疗器械许可证申请审查费用 加拿大
VIP免费
Health Santé
Canada Canada
Guidance Document
Fees for the Review of Medical Device
Licence Applications
Date adopted: 1997/05/01
Date posted: 2019/11/04
Canada
Health Canada is responsible for helping Canadians maintain and improve their health.It
ensures that high-quality health services are accessible,and works to reduce health risks.
Egalement disponible en francais sous le titre:
Ligne directrice:Frais pour Pexamen des demandes d'homologation des instruments médicaux
To obtain additional information,please contact:
Health Canada
Address Locator 0900C2
Ottawa,Ontario K1A OK9
Tel.:613-957-2991
Toll free:1-866-225-0709
Fax:613-941-5366
TTY:1-800-465-7735
E-mail:hc.publications-publications.sc@canada.ca
Her Majesty the Queen in Right of Canada,as represented by the Minister of Health,2019
Publication date:November 2019
This publication may be reproduced for personal or internal use only without permission
provided the source is fully acknowledged.
Cat.:H13-9/20-2019E-PDF
ISBN:978-0-660-27439-3
Pub.:180196
Fees for the Review of Medical Device Licence Applications |2
Document change log
Version
Guidance Document:Fees for
the Review of Medical Device
Licence Applications
Replaces
Guidance Document:Fees
for the Review of Medical
Device Licence Applications
Date
April 1,2020(posted
November 4,2019)
Date
November 20,2015
Date
Change
Location
(Section,
paragraph)
Nature of and/or Reason
for change
April 1,2020
(posted
November 4,
2019)
Content was updated
All
As of April 1,2020,new
fees along with a revised
fee policy willcome into
force requiring significant
changes to the guidance
document
November
20,2015
Administrative Change
S.2.2.2
As of November 9th,2015,
the Accounts Receivable
address has changed
摘要:
展开>>
收起<<
HealthSantéCanadaCanadaGuidanceDocumentFeesfortheReviewofMedicalDeviceLicenceApplicationsDateadopted:1997/05/01Dateposted:2019/11/04CanadaHealthCanadaisresponsibleforhelpingCanadiansmaintainandimprovetheirhealth.Itensuresthathigh-qualityhealthservicesareaccessible,andworkstoreducehealthrisks.Egaleme...
声明:如果您的权利被侵害,请联系我们的进行举报。
相关推荐
-
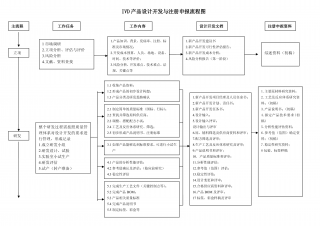
IVD产品设计开发以及注册申报流程图VIP免费
 2024-04-12 202
2024-04-12 202 -
医疗器械设计开发控制指南VIP免费

 2024-04-12 272
2024-04-12 272 -
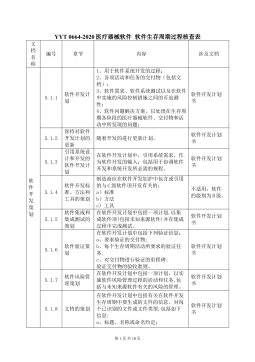
YY∕T 0664-2020医疗器械软件软件生存周期过程核查表VIP免费
 2024-04-12 276
2024-04-12 276 -
创新医疗器械注册申报流程

 2024-05-02 134
2024-05-02 134 -
20221028_医疗器械生产现场核查缺陷分析交流(江苏药省监局审核查验中心) (1)VIP免费

 2024-05-09 91
2024-05-09 91 -
医疗器械网络安全漏洞自评报告VIP专享

 2024-11-18 293
2024-11-18 293 -
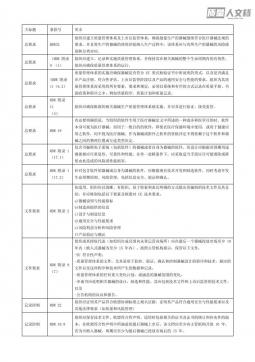
内审检查表 MDR法规VIP免费
 2025-04-07 242
2025-04-07 242 -
07.产品风险管理报告VIP免费

 2025-09-12 42
2025-09-12 42 -
06.可用性确认报告或可用性总结性测试报告VIP免费

 2025-09-12 64
2025-09-12 64 -
特定上市前提交审查的质量管理体系信息-2025草案_中英文版VIP专享

 2025-11-03 48
2025-11-03 48
作者:冒牌货
分类:专业资料
价格:60质量币
属性:15 页
大小:392.22KB
格式:PDF
时间:2026-01-08


