ISO 10993-3-2014 医疗器械生物学评价第3部分:遗传毒性、致癌性和生殖毒性试验(中英文版)
ISO 10993-3:2014(E) 中英文版
INTERNATIONAL
STANDARD
国际标准
Third edition
第3版
2014-10-01
Biologicalevaluationof medicaldevices —
Part3:Testsforgenotoxicity,
carcinogenicityandreproductivetoxicity
医疗器械生物学评价
第3部分:遗传毒性、致癌性和
生殖毒性试验
(中英文版)
2024年03月翻译
Reference number
ISO 10993-3:2014(E)
PAGE:1 CISO2014-Allrightsreserved
ISO 10993-3:2014(E) 中 英 文 版
目 录
Foreword 前言......................................................................................................................................................................................4
Introduction 介绍...............................................................................................................................................................................
8
1 .Scope 范围 ................................................................................................................................................................................... 10
2. Normative references 规范性引用文件............................................................................................................................... 10
3 .Terms and definitions 术语及定义...................................................................................................................................... 1
4 Requirements for test strategies 测试策略要求.............................................................................................................13
4.1 General 总则........................................................................................................................................................................... 13
4.2 Additional requirements for carcinogenicity testing 致癌性测试的附加要求...................................................... 14
4.3 Additional requirements for reproductive toxicity testing 生殖毒性试验的附加要求......................................15
5 Genotoxicity tests 遗传毒性试验....................................................................................................................................... 15
5.1 General 总则........................................................................................................................................................................... 15
5.2 Test strategy 测试策略..................................................................................................................................................... 15
5.3 Sample preparation 样品制备....................................................................................................................................... 20
6 Carcinogenicity tests 致癌性试验....................................................................................................................................... 21
6.1 General 总则.......................................................................................................................................................................... 21
6.2 Evaluation strategy 评估策略.......................................................................................................................................... 2
6.3 Sample preparation 样品制备....................................................................................................................................... 23
6.4 Test methods 试验方法 ................................................................................................................................................... 24
7 Reproductive and developmental toxicity tests 生殖和发育毒性试验................................................................. 26
7.1 General 总 则...........................................................................................................................................................................26
7.2 Test strategy 测试策略....................................................................................................................................................... 26
7.3 Sample preparation 样品制备....................................................................................................................................... 27
7.4 Test methods 试验方法......................................................................................................................................................28
8 Test report 测试报告...................................................................................................................................................................29
Annex A (informative ) Guidance on selecting an appropriate sample preparation procedure in genotoxicity
testing
附 录 A( 资 料 性 附 录 ) 遗 传毒 性 测 试 中 选 择 适 当样 品 制 备 程 序 的 指 南 ........................................................................... 30
Annex B(informative ) Flowchart for follow -up evaluation
附录B( 资 料 性 附录 ) 后 续 评估 流 程 图 ....................................................................................................................................... 4
Annex C ( informative ) Rationale of test systems
附 录C(资料性附录)测试系统的基本原理............................................................................................................................... 45
Annex D (informative ) Cell transformation test systems
附 录D(资料性附录)细胞转化试验系统................................................................................................................................... 48
PAGE:2 CISO2014-Allrightsreserved
ISO 10993-3:2014(E) 中 英 文 版
Annex E (normative ) Considerations for carcinogenicity studies performed as implantation studies
附 录E(规范性附录)作为植入研究进行致癌性研究的注意事项 .......................................................................................... 50
Annex F (informative ) In vitro tests for embryo toxicity
附录 F(资料性附录)胚胎毒性体外试验 ......................................................................................................................................52
Bibliography 参考文献 ..................................................................................................................................................................... 54
PAGE:3 CISO2014-Allrightsreserved
摘要:
展开>>
收起<<
ISO10993-3:2014(E)中英文版INTERNATIONALSTANDARD国际标准Thirdedition第3版2014-10-01Biologicalevaluationofmedicaldevices—Part3:Testsforgenotoxicity,carcinogenicityandreproductivetoxicity医疗器械生物学评价第3部分:遗传毒性、致癌性和生殖毒性试验(中英文版)2024年03月翻译ReferencenumberISO10993-3:2014(E)PAGE:1CISO2014-AllrightsreservedISO10993-3:2014(...
声明:如果您的权利被侵害,请联系我们的进行举报。
相关推荐
-
ASTM F3172-15 血管内医疗器械设计验证数量和样本容量选择指南

 2024-04-15 138
2024-04-15 138 -
ASTM-D-882-测量塑料薄膜和薄片材拉伸性能(

 2024-04-16 103
2024-04-16 103 -
ASTM F838-2015 确定用于液体过滤的膜过滤器的细菌滞留的标准试验方法

 2024-05-09 98
2024-05-09 98 -
ASTM F3064-21 Standard Specification for Normal Category Aeroplanes CertificationVIP免费

 2024-05-09 58
2024-05-09 58 -
ASTM D3195 D3195M-10(2015) Standard Practice for Rotameter CalibrationVIP免费

 2024-05-09 66
2024-05-09 66 -
ASTM D4169-22 运输集装箱和系统的性能测试1(中文版)VIP免费

 2025-09-09 56
2025-09-09 56 -
ASTM D4169 运输集装箱和设备性能试验的标准实施规范(中英文)VIP免费

 2025-09-09 42
2025-09-09 42 -
ASTME2500 确证方法--关键质量属性关键工艺参数关键方面VIP免费
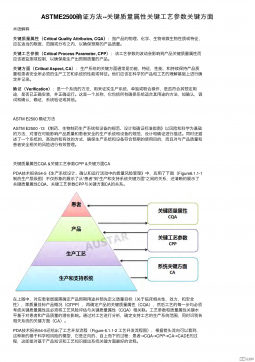
 2025-09-26 26
2025-09-26 26 -
ASTM E2500-25 药品的规格、设计与验证以及生物制药生产系统装备科学与基于风险的方法1(中文)VIP免费

 2025-11-21 86
2025-11-21 86 -
ASTM E3219-25 基于健康的暴露限值(HBEL)推导指南(中文)

 2025-12-17 82
2025-12-17 82
作者: 51zlzl
分类:法规规范
价格:200质量币
属性:65 页
大小:900.76KB
格式:PDF
时间:2025-11-20