ISO 14155-2020医疗器械临床实验管理规范
VIP免费
Clinical investigation of medical
devices for human subjects — Good
clinical practice
Investigation clinique des dispositifs médicaux pour sujets humains —
Bonne pratique clinique
© ISO 2020
INTERNATIONAL
STANDARD
ISO
14155
Third edition
2020-07
Reference number
ISO 14155:2020(E)
Copyright International Organization for Standardization
Provided by IHS Markit under license with Standards Council of Canada
Not for Resale, 08/01/2020 07:24:26 MDT
No reproduction or networking permitted without license from IHS
--`,``,,`,``,`,,`,,,,,,,,,````-`-`,,`,,`,`,,`---
ISO 14155:2020(E)
ii © ISO 2020 – All rights reserved
COPYRIGHT PROTECTED DOCUMENT
© ISO 2020
Website: www.iso.org
Copyright International Organization for Standardization
Provided by IHS Markit under license with Standards Council of Canada
Not for Resale, 08/01/2020 07:24:26 MDT
No reproduction or networking permitted without license from IHS
--`,``,,`,``,`,,`,,,,,,,,,````-`-`,,`,,`,`,,`---
ISO 14155:2020(E)
Foreword ..........................................................................................................................................................................................................................................v
1 Scope ................................................................................................................................................................................................................................. 1
2 Normative references ...................................................................................................................................................................................... 1
..................................................................................................................................................................................... 1
4 Summary of good clinical practice (GCP) principles ....................................................................................................... 9
5 Ethical considerations .................................................................................................................................................................................10
........................................................................................................................................................................................................ 10
....................................................................................................................................10
.................................................................................................................... 10
.............................................................................................................. 11
5.5 Responsibilities ...................................................................................................................................................................................11
...................................................................................................11
................................................................................................................................................................................... 11
................................................................................................................................................. 11
................................................................................................... 12
................................................................................................... 12
..................................................................... 12
................................................................................................................................................................. 12
...............................................................................................................................................................................13
................................................................................................................................................................................... 13
..................................................................................................... 13
........................................................................................14
..............................................................................................15
................................................................................................................................ 17
...........................................................................................................................................................17
6 Clinical investigation planning ...........................................................................................................................................................17
........................................................................................................................................................................................................ 17
..............................................................................................................................................................................
...................................................................................................................................................................................
.....
............................................................................................................................
....................................................................................
............................................................................................................................................
.....................................................................................................................................................
............................................................................................................................................................ 20
................................................................................................................................................................................... 20
........................................................................................................................................................ 21
......................................................................................................................................................................................... 21
.................................................................................................................................................................................................... 21
...................................................................................................................................21
7 Clinical investigation conduct ..............................................................................................................................................................22
........................................................................................................................................................................................................ 22
....................................................................................................................................................... 22
..................................................................................................................................................22
.........................................................................................................................22
............................................................................................................. 22
7.4.2 Adverse events ................................................................................................................................................................ 23
......................................................................................................................................................23
..................................................23
...................................................................................... 24
7.5.1 Amendments .................................................................................................................................................................... 24
....................................................................................................................................... 24
© ISO 2020 – All rights reserved iii
Contents
Copyright International Organization for Standardization
Provided by IHS Markit under license with Standards Council of Canada
Not for Resale, 08/01/2020 07:24:26 MDT
No reproduction or networking permitted without license from IHS
--`,``,,`,``,`,,`,,,,,,,,,````-`-`,,`,,`,`,,`---
摘要:
展开>>
收起<<
Clinicalinvestigationofmedicaldevicesforhumansubjects—GoodclinicalpracticeInvestigationcliniquedesdispositifsmédicauxpoursujetshumains—Bonnepratiqueclinique©ISO2020INTERNATIONALSTANDARDISO14155Thirdedition2020-07ReferencenumberISO14155:2020(E)CopyrightInternationalOrganizationforStandardizationProvi...
声明:如果您的权利被侵害,请联系我们的进行举报。
相关推荐
-
纯化水中国药典2020版与2025版GAP分析VIP免费
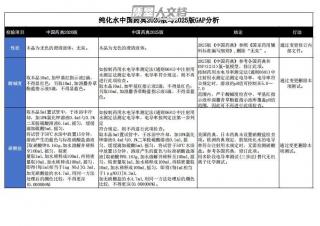
 2025-04-18 315
2025-04-18 315 -
无菌检查法对比表(2025版药典 VS 2020版药典)

 2025-09-27 402
2025-09-27 402 -
1101无菌检查法对比表(2025版药典 VS 2020版药典)
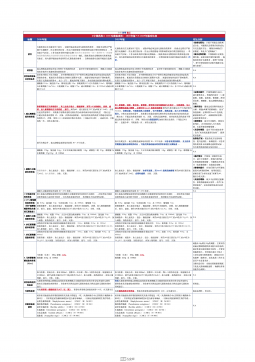
 2025-09-29 295
2025-09-29 295 -
1143 细菌内毒素检查法对比表(2025版药典 VS 2020版药典)

 2025-09-29 639
2025-09-29 639 -
ECA-污染控制策略指南(中英文)-202202VIP免费

 2025-11-04 68
2025-11-04 68 -
TR-26-2025-Sterilizing-Filtration-of-Liquids液体的灭菌过滤(中英文对照版)VIP专享

 2025-11-06 409
2025-11-06 409 -
TR-26-2025-Sterilizing-Filtration-of-Liquids液体的灭菌过滤(英文版)VIP免费

 2025-11-06 130
2025-11-06 130 -
PDA-TR49-生物清洁验证-中英文翻译

 2025-11-19 88
2025-11-19 88 -
硫酸艾玛昔替尼片(CXHS2300097)说明书VIP免费

 2025-11-26 185
2025-11-26 185 -
达格列净片说明书VIP免费

 2026-01-13 29
2026-01-13 29
作者:多多猪
分类:法规规范
价格:50质量币
属性:90 页
大小:1.33MB
格式:PDF
时间:2025-09-17


